The most common battery technology used for renewable energy systems is Lead-acid, in which lead plates are submerged in sulfuric acid. Lead acid batteries (while bulky) are relatively inexpensive and readily available compared to other battery types.
Lithium-ion battery technology encompasses a range of battery chemistries that use various Lithium compounds to store electrical energy. These batteries are much smaller and lighter than equivalent lead-acid batteries and can be charged and discharged faster and to greater extremes without suffering damage, decreasing the capacity required. Despite being significantly more expensive than lead-acid batteries, they can be the right choice when transportation costs and/or storage space is at a premium.
Battery Size
To properly design a battery bank, you need to account for the storage capacity required, the maximum discharge rate (the sum of all the loads which might be run simultaneously), the maximum charge rate (the current output from the solar array or wind turbine though the charge controller), and the minimum ambient temperature at which the batteries will be used. Whichever of these factors requires the largest capacity will dictate the size of the battery bank. The storage capacity of a battery – the amount of electrical energy it can hold – is usually expressed in amp-hours (Ah). Using one amp for 100 hours means 100 Ah have been used. A battery bank in a PV power system should have sufficient capacity to supply needed power during the longest expected period of cloudy weather. A lead-acid battery should be sized 20% to 50% larger than this amount, while a lithium-ion battery should be larger by at least 10% to 20%. If there is a source of on-demand backup power, such as a standby generator with a battery charger, the battery bank does not have to be sized for worst-case weather conditions.
Lead-Acid Batteries
Lead-acid batteries come in a wide variety of sizes and types, but the most important designation is whether they are deep cycle batteries or shallow cycle batteries.
Shallow cycle batteries, like the starting batteries in automobiles, are designed to supply a large amount of current for a short time and to stand mild overcharge without losing electrolyte; however, they cannot tolerate being deeply discharged. If they are repeatedly discharged to less than 50% of capacity, their useful life is dramatically shortened. Shallow cycle batteries are a poor choice for PV systems as they would be routinely discharged to the point of damage. Deep cycle batteries, on the other hand, are designed to be repeatedly discharged by as much as 80% of their capacity and are therefore a better choice for PV systems. Although they are designed to withstand deep cycling, these batteries will have a longer life if the cycles are shallower. Failing to fully recharge lead-acid batteries after each cycle or letting them stay in a discharged condition for more than a few hours will cause a permanent loss of capacity.
Sealed lead-acid batteries (gel cells and absorbed glass mat) are often referred to as maintenance-free because they never need watering or an equalization charge. This makes them well-suited for remote or unattended power systems. However, sealed batteries require very accurate regulation to prevent overcharge and over-discharge. Either of these conditions will dramatically shorten their lives.
Caring for Lead-Acid Batteries
Always use extreme caution when handling batteries and electrolyte (sulfuric acid). Wear appropriate personal protective equipment, including electrical and chemical resistant gloves with sleeves, goggles and acid-resistant clothing. "Battery acid" will instantly burn skin and eyes and destroy cotton and wool clothing.
Lead-acid batteries should always be recharged as soon as possible. The positive plates change from lead oxide when charged, to lead sulfate when discharged. The longer they remain in the lead sulfate state the more of the plate remains lead sulfate when the battery is recharged. The portion of the plates that become "sulfated" can no longer store energy. Batteries that are deeply discharged and then only partially charged on a regular basis often fail in less than one year. Check your batteries on a regular basis to be sure they are being charged. Use a hydrometer to check the specific gravity of your lead-acid batteries. If batteries are cycled very deeply and then recharged slowly, the specific gravity reading will be lower because of incomplete mixing of electrolyte.
Check the electrolyte level in wet-cell, or "flooded" batteries, at least once every 3 months and top-off each cell with distilled water. Do not add water to discharged batteries! Electrolyte is absorbed when batteries are discharged, so if you add water at this time and then recharge the battery, electrolyte will overflow and create a safety hazard. Keep the tops of your batteries clean and check that cables are tight. Do not tighten or remove cables while charging or discharging! Any spark around batteries can cause a hydrogen explosion inside the case and potentially ignite a fire or an even larger explosion if the batteries are not properly vented.
An "equalization" charge should be performed on flooded batteries whenever cells show a variation of 0.05 or more in specific gravity from each other. This is a long steady overcharge, bringing the battery to a gassing or bubbling state. Do not equalize sealed or gel-type batteries! With proper care, lead-acid batteries will have a long service life and work very well in almost any power system.
Battery warranties do NOT cover damage due to poor maintenance or loss of capacity from sulfation.
Battery Sizing Worksheet
Use this worksheet to determine what size battery bank is required for your system. Battery size, or capacity, is measured in amp-hours. Battery voltage is determined by the number of "cells" in series. All lead-acid battery cells have a nominal output of 2 VDC. Actual cell voltage varies from about 1.7 VDC at full discharge to 2.4 VDC at full charge. 12 VDC lead-acid batteries are made of 6 separate cells in one case. 6 VDC batteries are made of 3 cells in one case. Putting battery cells in parallel increases amp-hour capacity, but does not change voltage.
| Step 1: | Total average amp-hours per day required (see Off-Grid Load Worksheet) |
| Step 2: |
Maximum number of continuous cloudy days expected in your area |
| Step 3: |
Multiply Step 1 result by Step 2 result |
| Step 4: |
Divide Step 3 result by 0.5 to 0.8 for discharge reserve: 0.5 will maintain a 50% reserve and maximize battery life 0.8 will maintain an 20% reserve and minimize battery bank size |
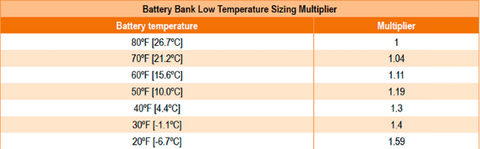
| Step 5: | If you are using a lead acid battery, select the multiplier from the table below that corresponds to the battery's wintertime average ambient temperature |
| Step 6: |
Maximum current that will be drawn by the loads for 10 minutes or more |
| Step 7: |
Maximum output current (in Amps) of PV array or other battery charger |
| Step 8: |
Multiply the Step 7 result by 10 |
| Step 9: |
Amp-hours from Step 4, 6 or 8, whichever is greatest |
| Step 10: |
Multiply the Step 5 results and step 9 to get required amp-hour capacity |
| Step 11: |
Capacity of preferred battery in Amp-hours |
| Step 12: |
Divide Step 10 result by Step 11 result and round up to a whole number. This is the number of parallel strings required |
| Step 13: | Divide the system voltage (12, 24, or 48) by the voltage of the chosen battery (2 V, 6 V or 12 V) to determine the number of batteries in each string |
| Step 14: |
Multiply the Step 12 result by the step 13 result to determine the total number of batteries needed |
Battery State-of-Charge
Battery state-of-charge (SOC) can be measured by an amp-hour meter, voltage or by specific gravity. Some care and knowledge is required to interpret state-of-charge from voltage or specific gravity readings. We recommend amp-hour meters for all systems with batteries. An amp-hour meter is like a fuel gauge for batteries and provides all the information needed to keep batteries charged. At a glance, the user can see system voltage, current, and battery condition (see Meters and Monitoring).
Measuring Battery State-of-Charge
Battery voltage will vary for the same state-of-charge depending on whether the battery is being charged or discharged, and what the current is in relation to the size of the battery. The table below shows typical battery voltages at each state-of-charge for various battery conditions in flooded cell lead-acid batteries. Voltage varies with temperature. While charging, a lower temperature will increase battery voltage. Full-charge voltage on a 12 VDC battery is 0.9 VDC higher at 32°F than at 70°F. While discharging, a higher temperature will increase battery voltage. There is little temperature effect while a battery is idle.

Hydrometers
A hydrometer is very accurate at measuring battery state-of-charge if you measure the electrolyte near the plates. Unfortunately, you can only measure the electrolyte at the top of the battery, which is not always near the plates. When a battery is being charged or discharged, a chemical reaction takes place at the border between the lead plates and the electrolyte. The electrolyte changes from water to sulfuric
acid while charging. The acid becomes stronger, increasing the specific gravity, as the battery charges. Near the end of the charging cycle, gas bubbles rising through the acid stir the fluid. It takes several hours for the electrolyte to mix so that you get an accurate reading at the top of the battery. Always try to take readings after the battery has been idle or slowly discharging for some time.
Hydrometer Readings
This table shows the battery state-of-charge corresponding to various specific gravities for a battery bank in an ambient temperature of 75°F.

Battery Wiring Diagrams
The diagrams below show typical 12 VDC, 24 VDC and 48 VDC battery wiring configurations. Batteries can deliver extremely high current. Always install fuse protection on any positive wiring connected to batteries.